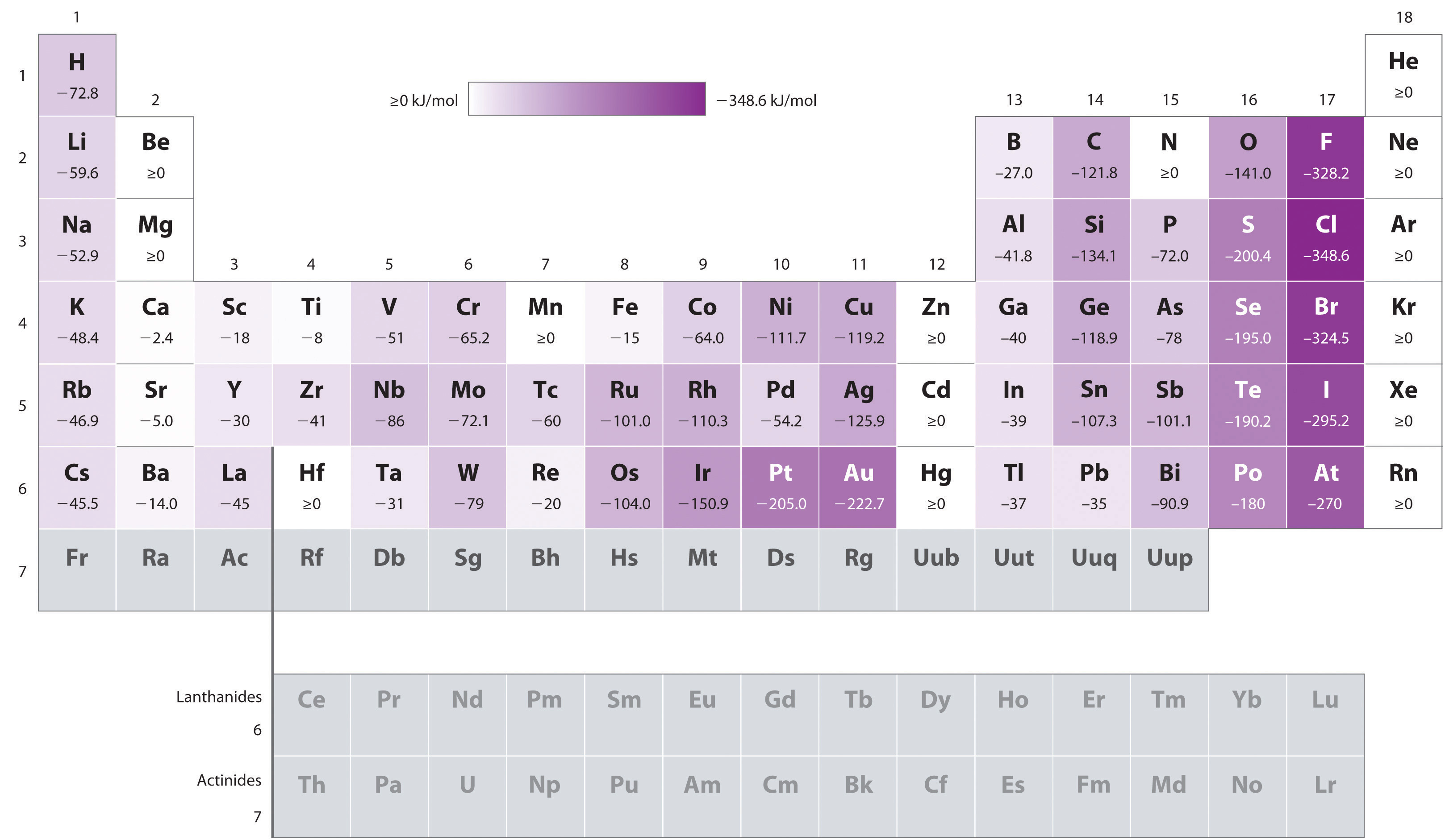
Chart Of Electron Affinity
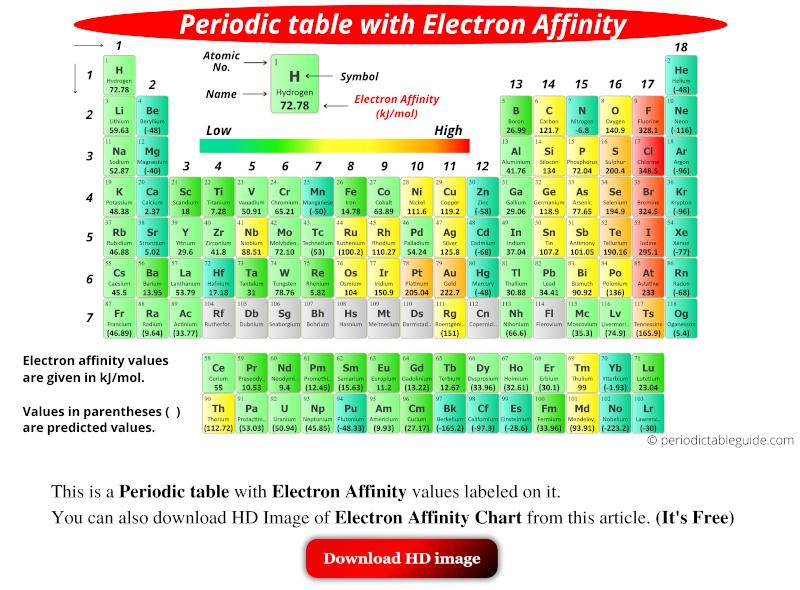
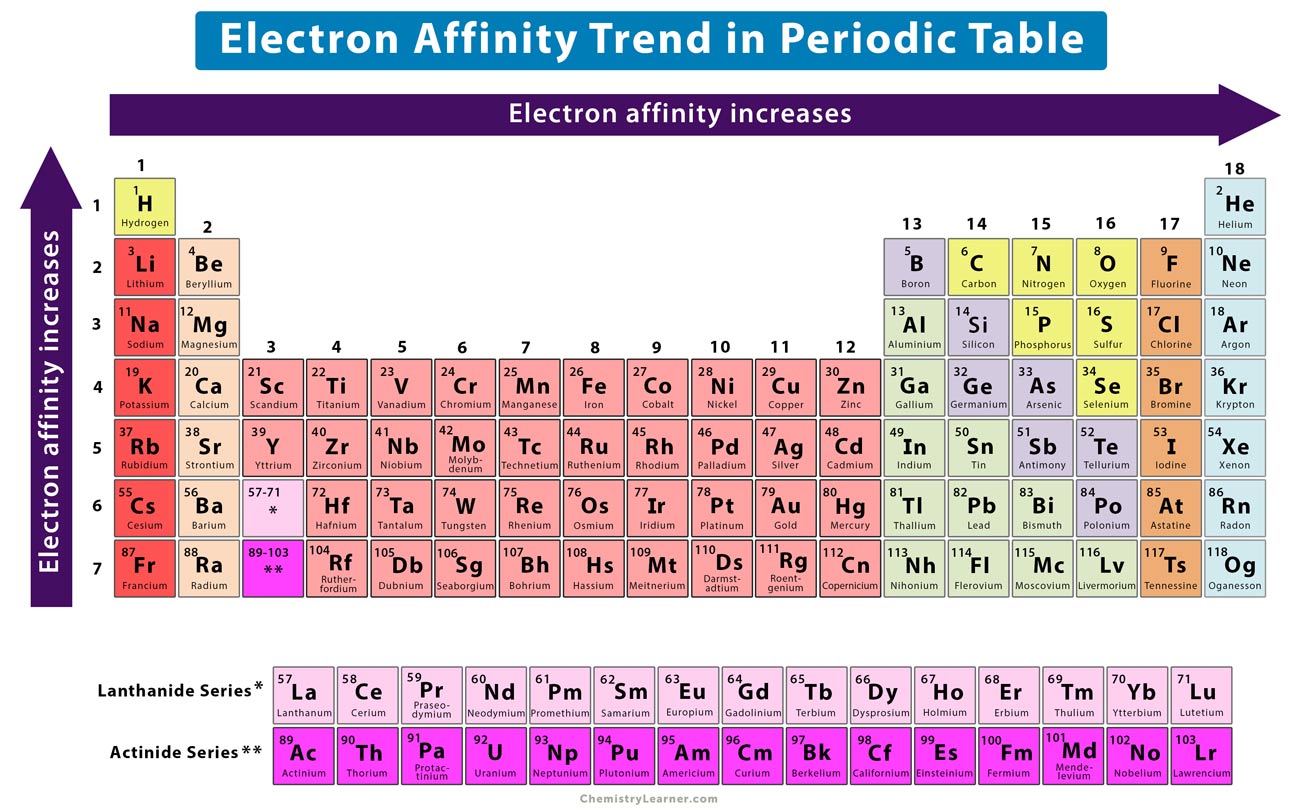
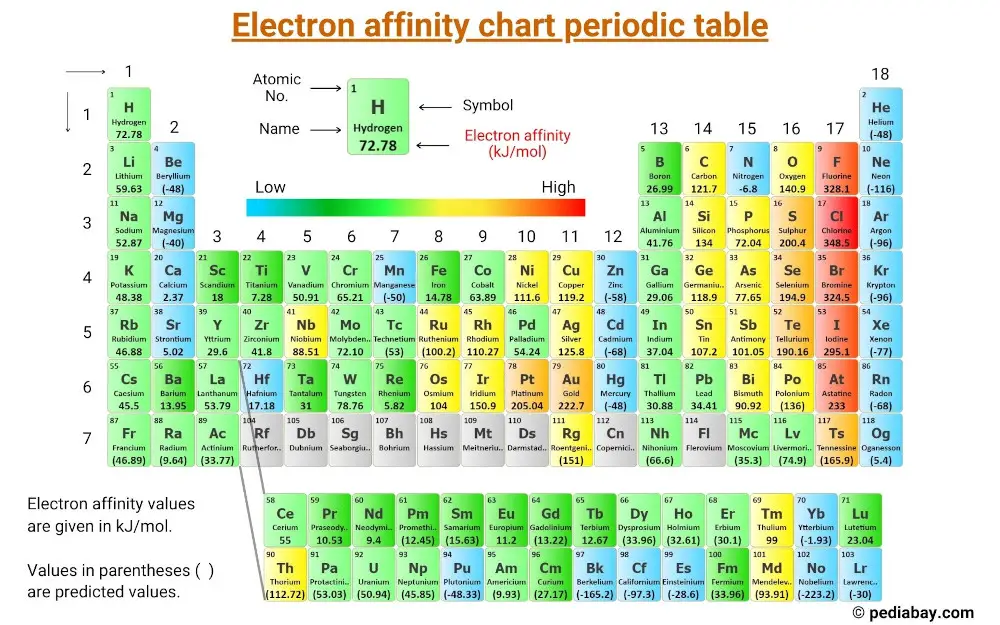
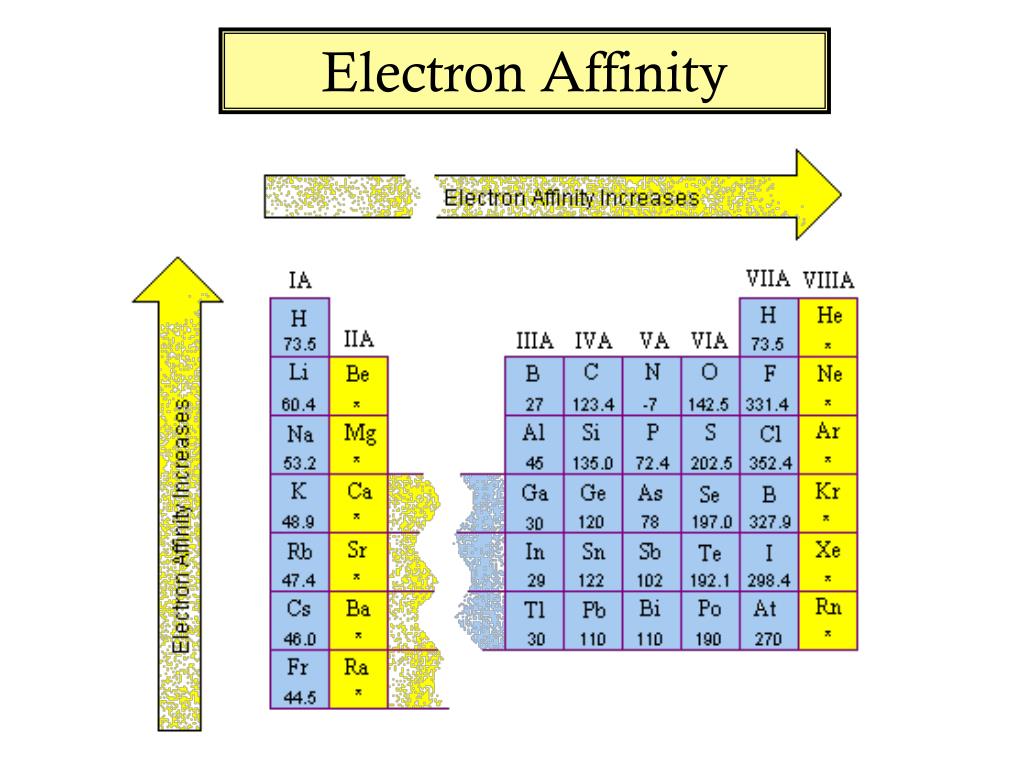
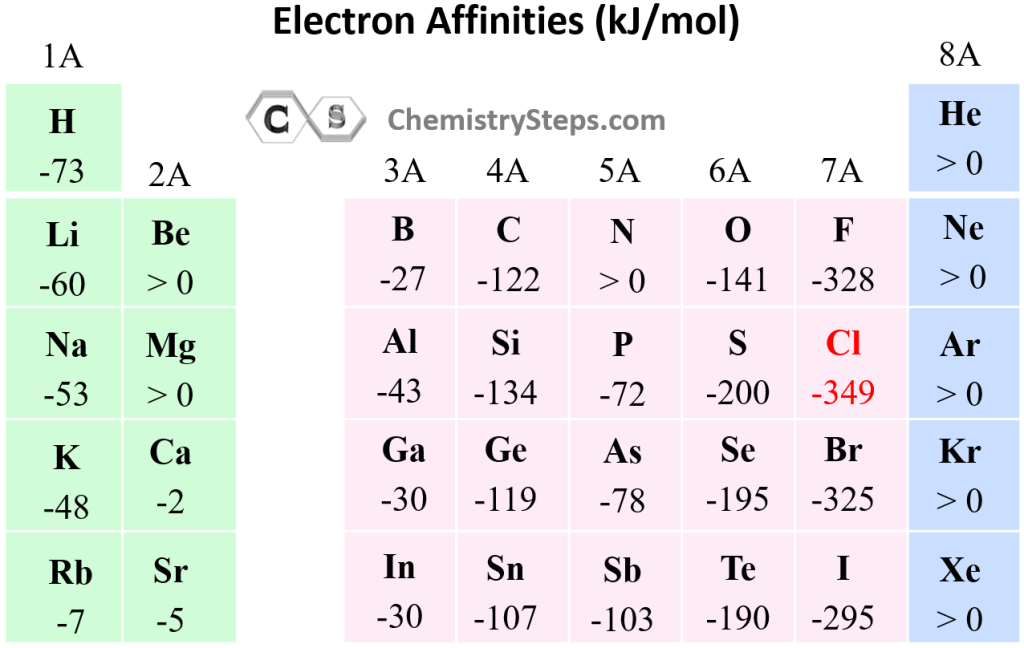
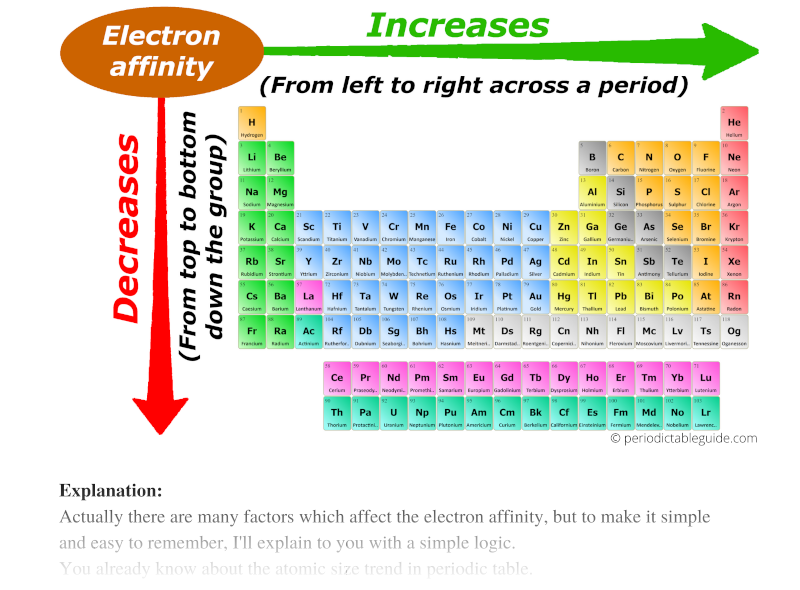
Chart Of Electron Affinity - Web electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion. Web electron affinity can be defined in two equivalent ways. So the more negative the electron affinity the more favourable the electron addition process is. Electron affinity is the energy change when an atom gains an electron. Web the electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. Web the electron affinity (e ea) of an atom or molecule is defined as the amount of energy release when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. For most elements, except noble gases, this is an exothermic process. Web the most common units for electron affinity are kilojoules per mole (kj/mol) or electronvolts (ev). Electron affinities decrease from top to bottom down groups. First electron affinities have negative values. Web the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion. The equivalent more common definition is the energy released (e initial+ e final) when an additional electron is attached to a neutral atom or molecule. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. First, as the energy that is released by adding an electron to an isolated gaseous atom. Web the most common units for electron affinity are kilojoules per mole (kj/mol) or electronvolts (ev). In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. Web the electron affinity (ea) is the energy released to add an electron to an elements in the gaseous state. Web electron affinity can be defined in two equivalent ways. Electron affinities decrease from top to bottom down groups. Web the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Below is a visual representation of electron affinity trends throughout the periodic table. Web the energy change that occurs when a neutral atom gains an electron is called its electron affinity. First, as the energy that is released by adding an electron to an isolated gaseous atom. Web table shows electron affinity (i.e. Web electron affinity can be defined in. How to find electron affinity Small numbers indicate that a less stable negative ion is formed. Web periodic table with electron affinity. Web electron affinity chart for all the elements of periodic table is shown in the below table. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest. Web the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Complete and detailed technical data about the element $$$elementname$$$ in the periodic table. Electron affinity (ev) electron affinity (kj/mol) 1. Web the electron affinity (ea) is the energy released to add an electron. Web electron affinity chart for all the elements are given below. Web the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Web the electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral. Web the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Web electron affinity can be defined in two equivalent ways. Web table shows electron affinity (i.e. The first electron affinities of the group 7 elements. Web electron affinity chart for all the elements. In general successive electron affinity increase in magnitude ea 1 < ea 2 < ea 3 and so on. So the more negative the electron affinity the more favourable the electron addition process is. Web the electron affinity (e ea) of an atom or molecule is defined as the amount of energy release when an electron attaches to a neutral. Web the electron affinity (e ea) of an atom or molecule is defined as the amount of energy release when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. So the more negative the electron affinity the more favourable the electron addition process is. Below is a visual representation of electron affinity. Web table shows electron affinity (i.e. Web electron affinity is the energy change that results from adding an electron to a gaseous atom. Electron affinity is the energy change when an atom gains an electron. Web the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the. Web electron affinity chart for all the elements of periodic table is shown in the below table. First electron affinities have negative values. The equivalent more common definition is the energy released (e initial+ e final) when an additional electron is attached to a neutral atom or molecule. How to find electron affinity Electron affinity also applies to molecules, in. Web electron affinity is the energy change that results from adding an electron to a gaseous atom. Web explore how electron affinity changes with atomic number in the periodic table of elements via interactive plots. As discussed, electron affinities increase from left to right across periods; You can also get the periodic table labeled with electron affinity values of elements.. Web electron affinity can be defined in two equivalent ways. Web electron affinity chart for all the elements of periodic table is shown in the below table. So the more negative the electron affinity the more favourable the electron addition process is. First, as the energy that is released by adding an electron to an isolated gaseous atom. The first electron affinities of the group 7 elements. Web periodic table with electron affinity. The figure below shows electron affinities in \(\ce{kj/mol}\) for the representative elements. By convention, the negative sign shows a release of energy. Web the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion. In general successive electron affinity increase in magnitude ea 1 < ea 2 < ea 3 and so on. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. Complete and detailed technical data about the element $$$elementname$$$ in the periodic table. Web the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. Web the energy change that occurs when a neutral atom gains an electron is called its electron affinity. Electron affinity also applies to molecules, in some cases. Web the electron affinity (ea) is the energy released to add an electron to an elements in the gaseous state.How about electron affinity? Electron affinity, Ionization energy
Electron Affinity Chart (Labeled Periodic table + List)
Electron Affinity Definition, Chart & Trend in Periodic Table
Electron Affinity Chart of Elements (With Periodic Table) Pediabay
PPT Chapter 4 The Periodic Table PowerPoint Presentation, free
Electron Affinity Chemistry Steps
Electron Affinity Trend and Definition
All Periodic Trends in Periodic Table (Explained with Image)
Periodic Behavior Presentation Chemistry
1.1.2.4 Electron Affinity Chemistry LibreTexts
Web The Electron Affinity (E Ea) Of An Atom Or Molecule Is Defined As The Amount Of Energy Release When An Electron Attaches To A Neutral Atom Or Molecule In The Gaseous State To Form An Anion.
You Can Also Get The Periodic Table Labeled With Electron Affinity Values Of Elements.
When Energy Is Released In A Chemical Reaction Or Process, That Energy Is Expressed As A Negative Number.
The Second (Reverse) Definition Is That Electron Affinity Is The Energy Required To Remove An Electron From A Singly Charged Gaseous Negative Ion.
Related Post:


.PNG)