Ate Ite Ide Chart
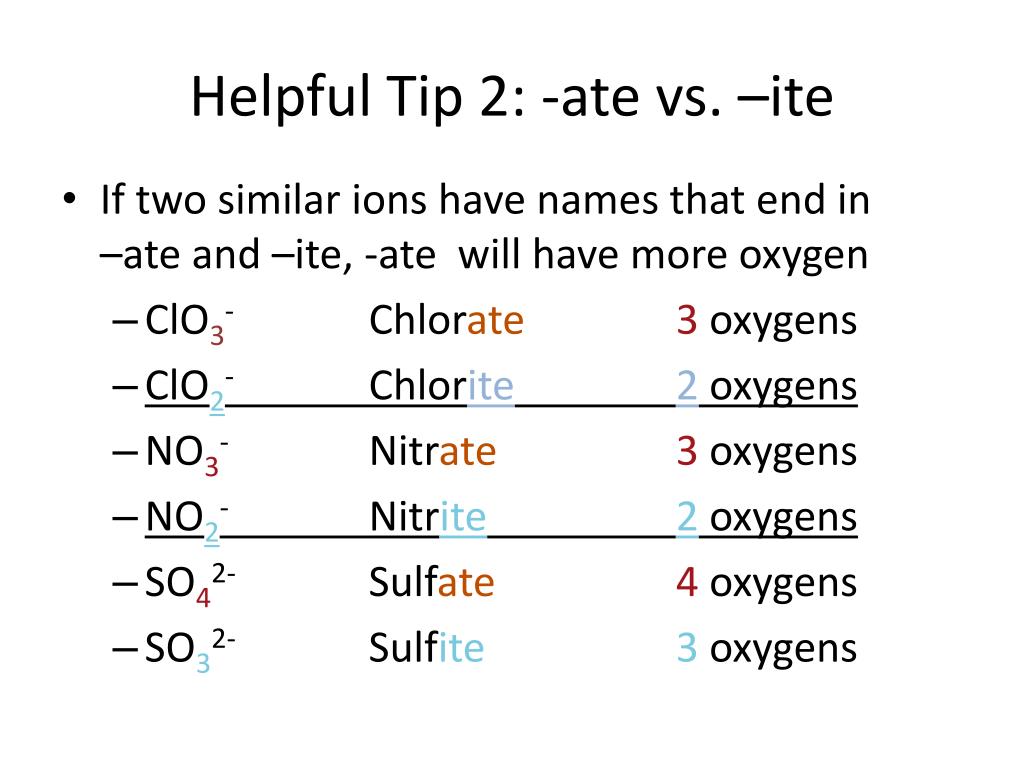
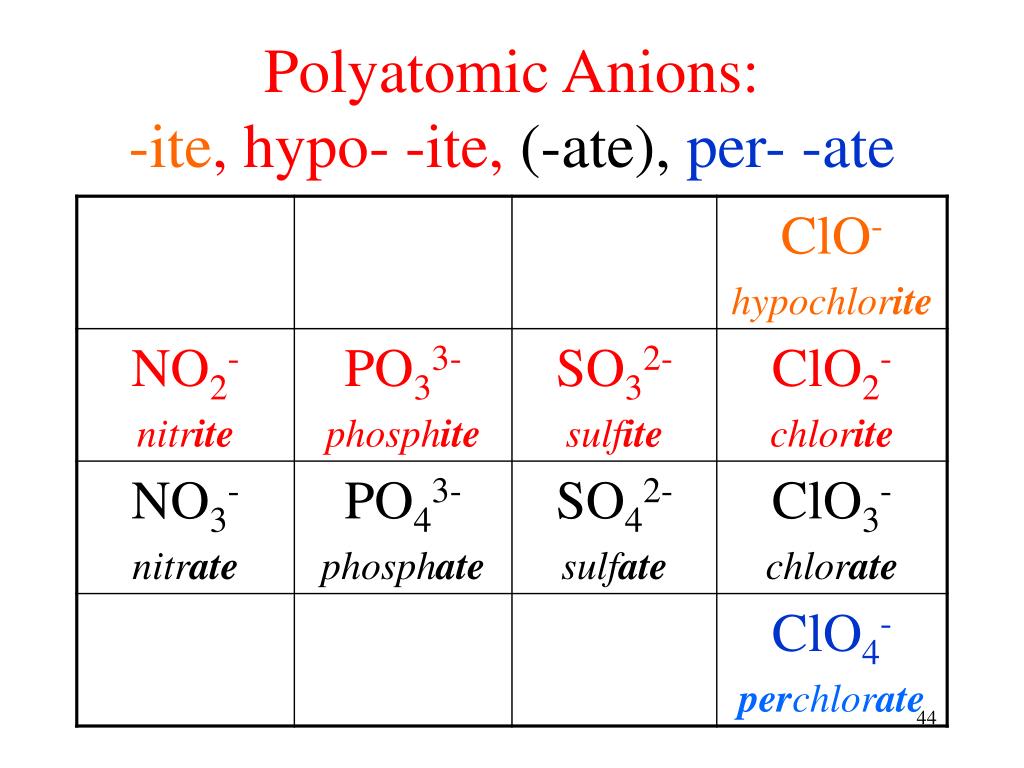
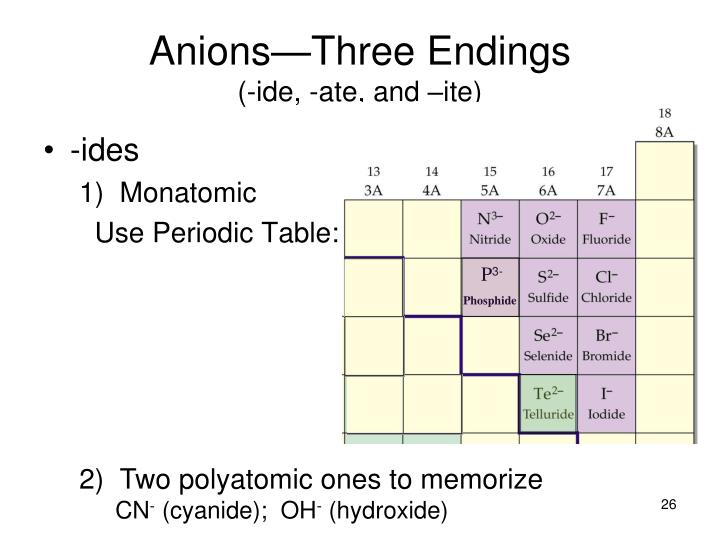
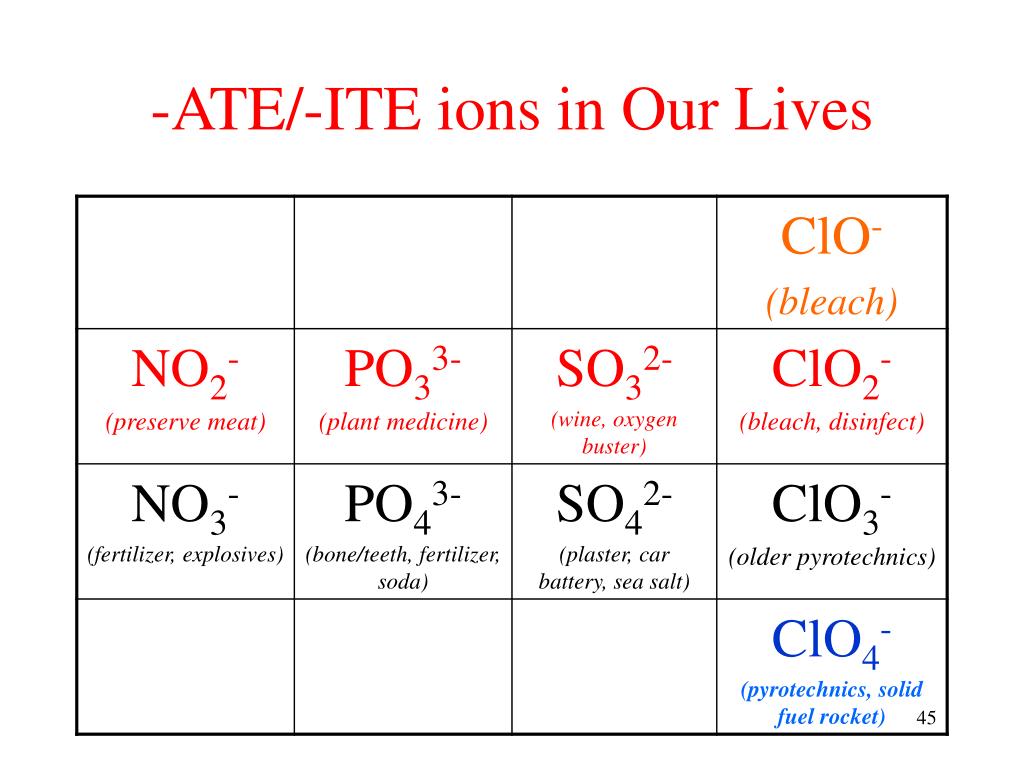
Ate Ite Ide Chart - Let's say below is what you see written: Web terms in this set (30) study with quizlet and memorize flashcards containing terms like ammonium, acetate, bromate and more. When an element forms two. These anions are called oxyanions. For example, chlorine forms a chloride ion, so nacl is sodium chloride. So, no2 is nitrite, and. Some polyatomic anions contain oxygen. Web flow chart for naming simple inorganic compounds. So no2 would be nitrite, and no3 would be. It doesn't denote a specific number. Web terms in this set (30) study with quizlet and memorize flashcards containing terms like ammonium, acetate, bromate and more. So, no2 is nitrite, and. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: Let's say below is what you see written: So no2 would be nitrite, and no3 would be. These anions are called oxyanions. Some polyatomic anions contain oxygen. Web flow chart for naming simple inorganic compounds. Web so you see, there's a big difference between ending with ide or with ate. The flowchart is adapted from p. For example, chlorine forms a chloride ion, so nacl is sodium chloride. Web flow chart for naming simple inorganic compounds. So no2 would be nitrite, and no3 would be. The flowchart is adapted from p. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: These anions are called oxyanions. Some polyatomic anions contain oxygen. Web flow chart for naming simple inorganic compounds. For example, chlorine forms a chloride ion, so nacl is sodium chloride. Web terms in this set (30) study with quizlet and memorize flashcards containing terms like ammonium, acetate, bromate and more. Some polyatomic anions contain oxygen. So no2 would be nitrite, and no3 would be. For example, chlorine forms a chloride ion, so nacl is sodium chloride. These anions are called oxyanions. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: For example, chlorine forms a chloride ion, so nacl is sodium chloride. When an element forms two. So no2 would be nitrite, and no3 would be. The flowchart is adapted from p. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: Web terms in this set (30) study with quizlet and memorize flashcards containing terms like ammonium, acetate, bromate and more. Let's say below is what you see written: Web flow chart for naming simple inorganic compounds. Some polyatomic anions contain oxygen. Learning a language means paying attention to patterns. Let's say below is what you see written: Learning a language means paying attention to patterns. When an element forms two. Web terms in this set (30) study with quizlet and memorize flashcards containing terms like ammonium, acetate, bromate and more. It is the suffix of the. It is the suffix of the. For example, chlorine forms a chloride ion, so nacl is sodium chloride. The flowchart is adapted from p. Web so you see, there's a big difference between ending with ide or with ate. These anions are called oxyanions. Learning a language means paying attention to patterns. Let's say below is what you see written: The flowchart is adapted from p. For example, chlorine forms a chloride ion, so nacl is sodium chloride. It is the suffix of the. So, no2 is nitrite, and. Some polyatomic anions contain oxygen. The flowchart is adapted from p. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: Web flow chart for naming simple inorganic compounds. Web so you see, there's a big difference between ending with ide or with ate. When an element forms two. Web flow chart for naming simple inorganic compounds. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: It doesn't denote a specific number. When an element forms two. These anions are called oxyanions. So, no2 is nitrite, and. Some polyatomic anions contain oxygen. For example, chlorine forms a chloride ion, so nacl is sodium chloride. Ending (suffix) is only for polyatomic anions that contain oxygen, when the polyatomic ion is contained in the following: Web flow chart for naming simple inorganic compounds. The flowchart is adapted from p. Web so you see, there's a big difference between ending with ide or with ate. It doesn't denote a specific number. It is the suffix of the. Let's say below is what you see written:Ate Ite Ide Chart
ide ite ate YouTube
Formula Making of Anions with suffix ide, ate, ite ( With Trick
PPT Chapter 6 “Chemical Names and Formulas” PowerPoint Presentation
PPT Guide to Naming Ionic Compounds PowerPoint Presentation, free
PPT Molecules and Compounds Nomenclature PowerPoint Presentation
PPT Breakdown of Topics PowerPoint Presentation ID6209332
HOW TO USE NOMENCLATURE YouTube
PPT Molecules and Compounds Nomenclature PowerPoint Presentation
Ate Ite Ide Chart
So No2 Would Be Nitrite, And No3 Would Be.
Learning A Language Means Paying Attention To Patterns.
Web Terms In This Set (30) Study With Quizlet And Memorize Flashcards Containing Terms Like Ammonium, Acetate, Bromate And More.
Related Post: