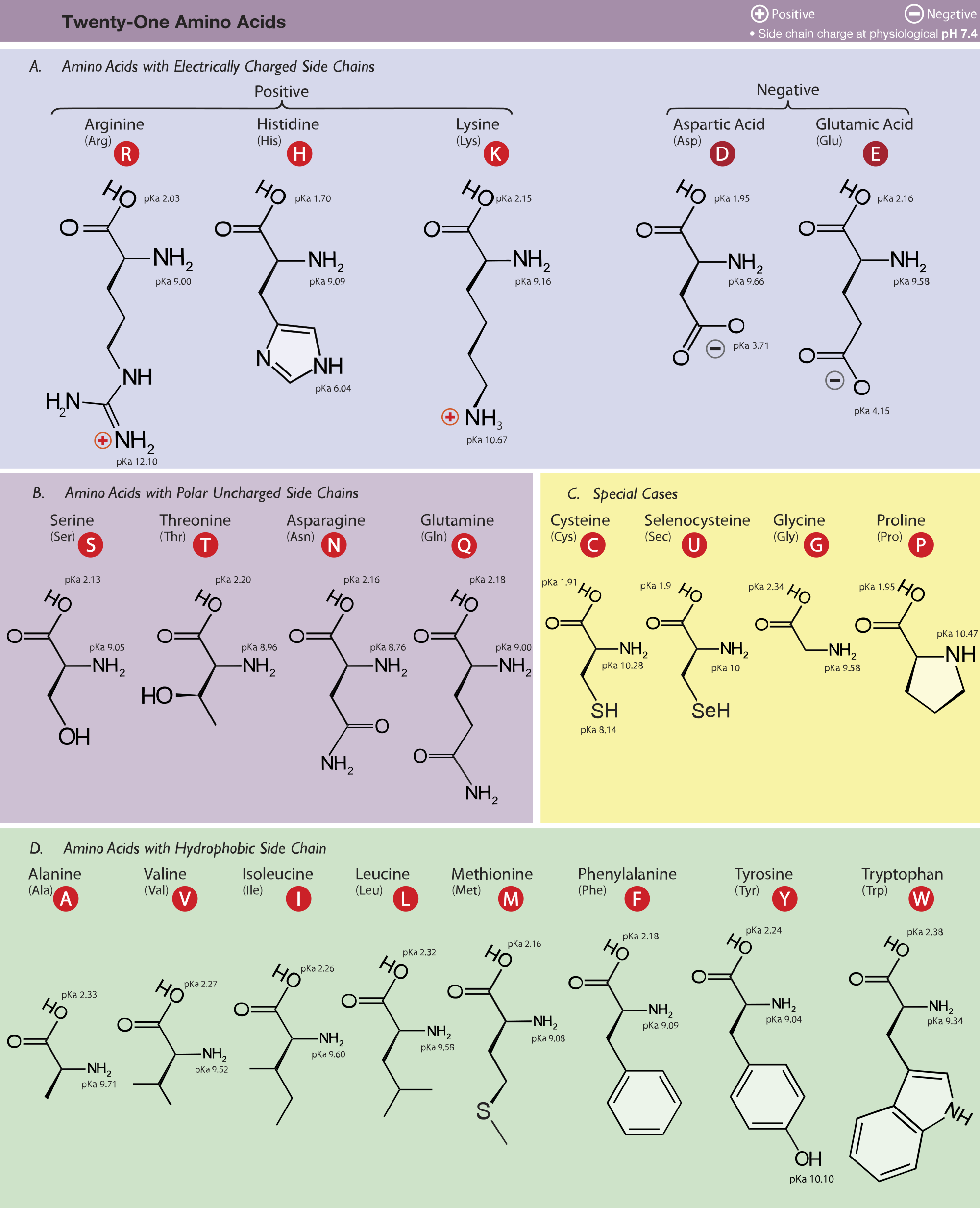
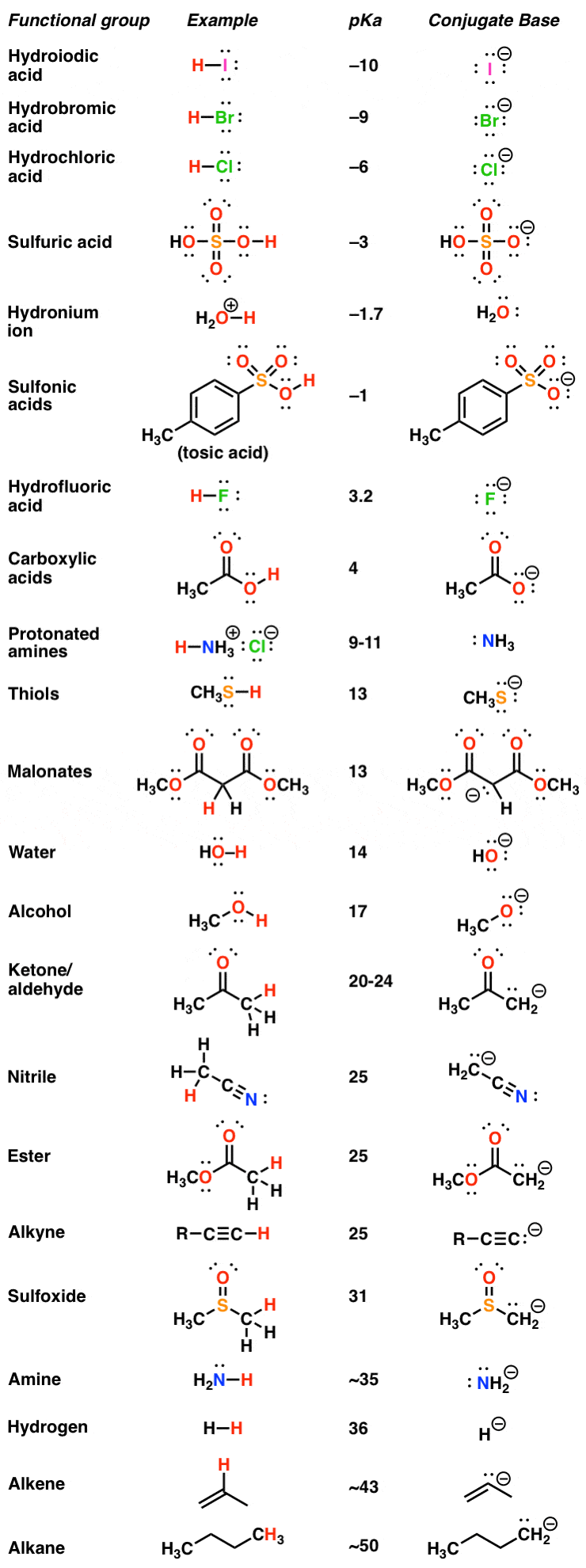
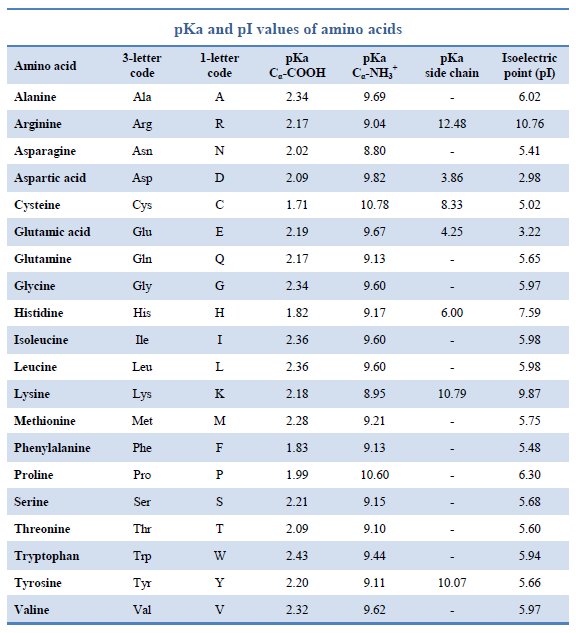
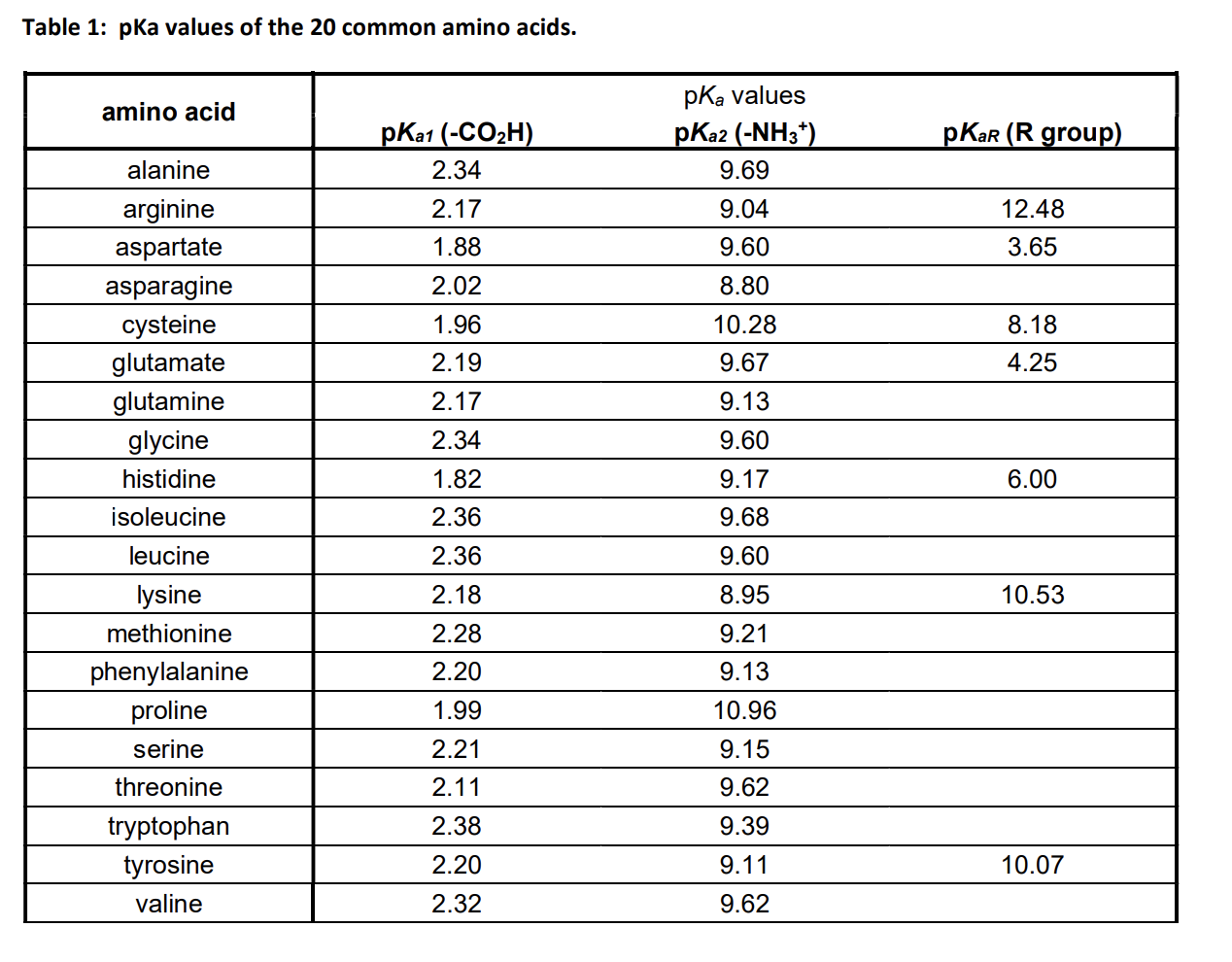
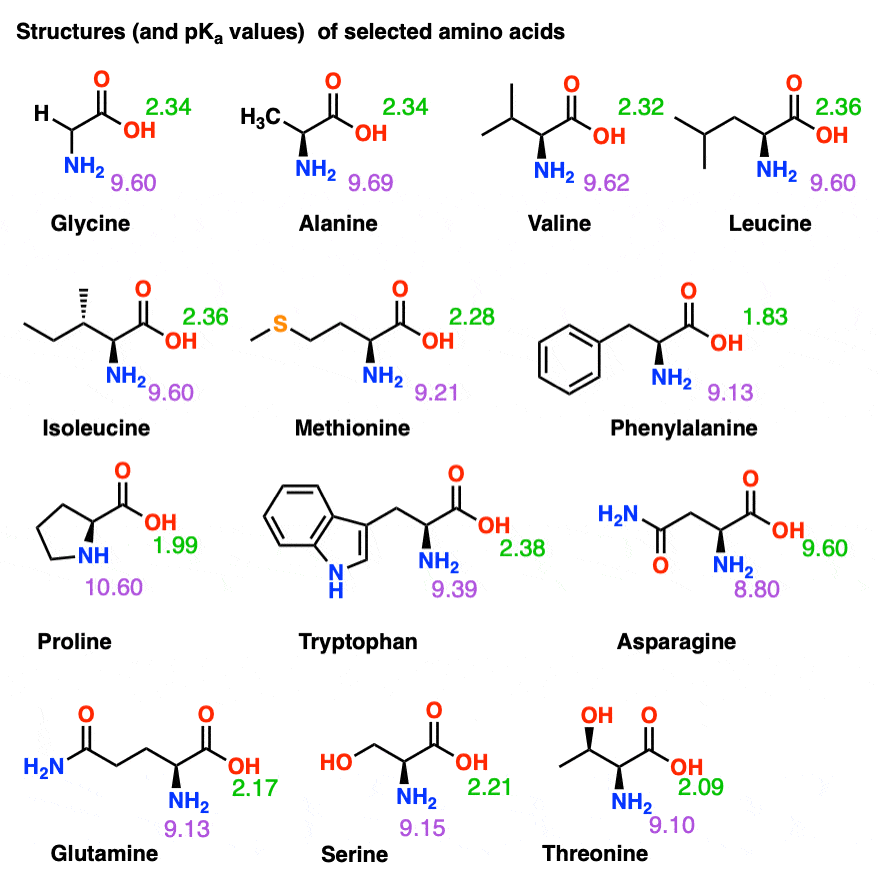
Amino Acid Pka Chart
Amino Acid Pka Chart - They contain an amino group, carboxylic acid group, alpha carbon, and side chain. Web basic molecules gain protons and acidic molecules donate protons. Web pka and electrical properties of amino acids. The isoelectric points range from 5.5 to 6.2. Web kainoid synthases are key enzymes in the biosynthesis of kainoids. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Because every nucleophile is potentially a base, and vice versa. Web most biochemistry courses will require you to know the following: It represents the negative logarithm of the acid. Amino acids have −cooh − cooh group that is acidic with pk a 2. Web all amino acids have the same basic structure, which is shown in figure 2.1. The ammonium holds the proton more tightly than does the acid. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Because every nucleophile is potentially a base, and vice versa. At the “center” of each amino acid is a carbon called the α carbon and attached to it. Web table of pk a and pi values. Web 20 amino acids and their functions, structures, names, properties, classifications. Most amino acids have a chiral carbon, which. If you have a reaction where it looks like you might get sn2 or e2,. If a molecule is a base or an acid, depends on their functional groups. Web only the guanidine group (hnc (nh2)2) in arg (pka = 12.5) and amine group in lys (pka = 10.5) will accept a proton and exist with an overall charge of +1 at physiological ph. Web table \(\pageindex{2}\) shows the standard pk a values for the amino acids and can be used to predict the ionization/charge status of amino acids. Web basic molecules gain protons and acidic molecules donate protons. Web kainoid synthases are key enzymes in the biosynthesis of kainoids. Web most biochemistry courses will require you to know the following: If you have a reaction where it looks like you might get sn2 or e2,. If a molecule is a base or an acid, depends on their functional. Amino acid pka and pi values Web kainoid synthases are key enzymes in the biosynthesis of kainoids. Web table of contents. The isoelectric point, pi, is the ph at which negative and positive charges are balanced. The ammonium holds the proton more tightly than does the acid. In organic chemistry, pka is a measure of the acidity or basicity of a compound. If you have a reaction where it looks like you might get sn2 or e2,. Certain functional groups give away. Most amino acids have a chiral carbon, which. At the “center” of each amino acid is a carbon called the α carbon and attached to. Because every nucleophile is potentially a base, and vice versa. Web amino acids are the building blocks of proteins. Amino acids have −cooh − cooh group that is acidic with pk a 2. Web all amino acids have the same basic structure, which is shown in figure 2.1. Web the pka of the acid is near 5, and the pka. Web amino acids are the building blocks of proteins. The ammonium holds the proton more tightly than does the acid. If you have a reaction where it looks like you might get sn2 or e2,. Amino acid pka and pi values At neutral ph the amino. Most amino acids have a chiral carbon, which. In organic chemistry, pka is a measure of the acidity or basicity of a compound. Web why are pkas so important? The proton stays on the nitrogen. Web kainoid synthases are key enzymes in the biosynthesis of kainoids. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Web most biochemistry courses will require you to know the following: Web table of pk a and pi values. Web basic molecules gain protons and acidic molecules donate protons. Web 20 amino acids and their functions, structures, names, properties,. Amino acid pka and pi values Web most biochemistry courses will require you to know the following: Web 20 amino acids and their functions, structures, names, properties, classifications. The isoelectric point, pi, is the ph at which negative and positive charges are balanced. Web pka and electrical properties of amino acids. Web most biochemistry courses will require you to know the following: If you have a reaction where it looks like you might get sn2 or e2,. At neutral ph the amino. Most amino acids have a chiral carbon, which. Web only the guanidine group (hnc (nh2)2) in arg (pka = 12.5) and amine group in lys (pka = 10.5) will. Most amino acids have a chiral carbon, which. Web table of pk a and pi values. In organic chemistry, pka is a measure of the acidity or basicity of a compound. Web 20 amino acids and their functions, structures, names, properties, classifications. Amino acids have −cooh − cooh group that is acidic with pk a 2. It represents the negative logarithm of the acid. Web this is why the carboxylic acid groups of amino acids have a lower pka value of around 2, while acetic acid has a pka value of 4.76. For the 13 amino acids with a neutral side. Refer to the charts and structures below to explore amino acid properties,. Web only the guanidine group (hnc (nh2)2) in arg (pka = 12.5) and amine group in lys (pka = 10.5) will accept a proton and exist with an overall charge of +1 at physiological ph. Web the pka of the acid is near 5, and the pka of the ammonium is near 9. Amino acid pka and pi values Web table \(\pageindex{2}\) shows the standard pk a values for the amino acids and can be used to predict the ionization/charge status of amino acids and their resulting. The proton stays on the nitrogen. Web the r group for each of the amino acids will differ in structure, electrical charge, and polarity. Web most biochemistry courses will require you to know the following:Chapter 2 Protein Structure Chemistry
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic
[Infographic] Comprehensive pKa Chart r/chemistry
Amino Acid Study Guide Structure and Function Albert.io
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry
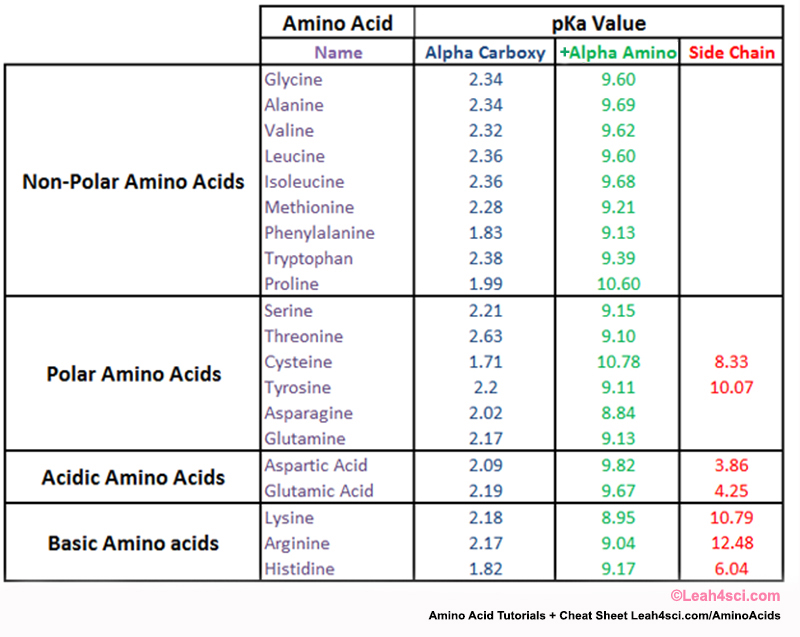
Amino acid properties
pKa Table
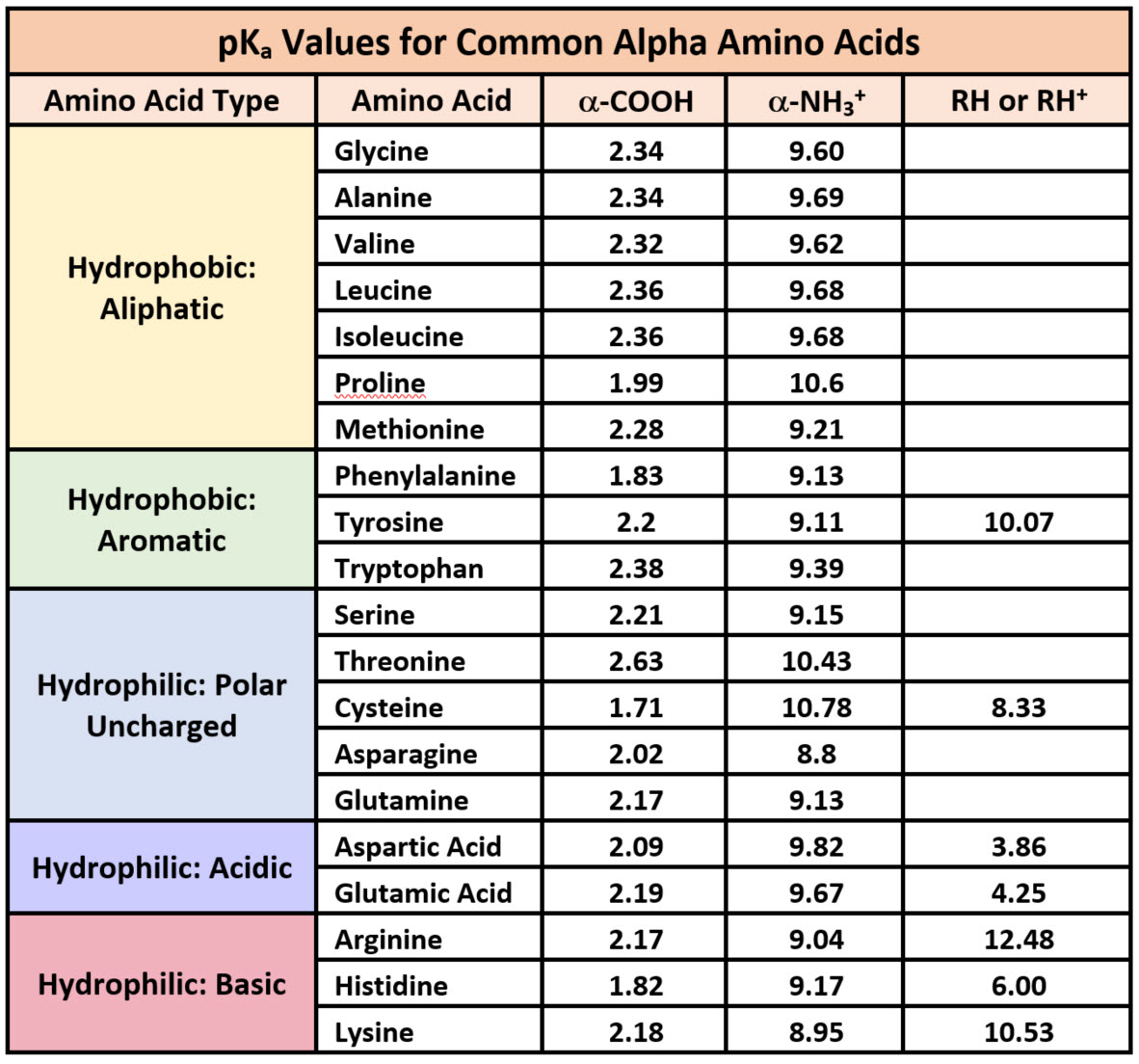
Solved Table 1 pKa values of the 20 common amino acids.
Isoelectric Points of Amino Acids (and How To Calculate Them) Master
Web All Amino Acids Have The Same Basic Structure, Which Is Shown In Figure 2.1.
Web Table Of Contents.
If You Have A Reaction Where It Looks Like You Might Get Sn2 Or E2,.
Web The Isoelectric Point Of An Amino Acid Is The Ph At Which The Amino Acid Has A Neutral Charge.
Related Post:
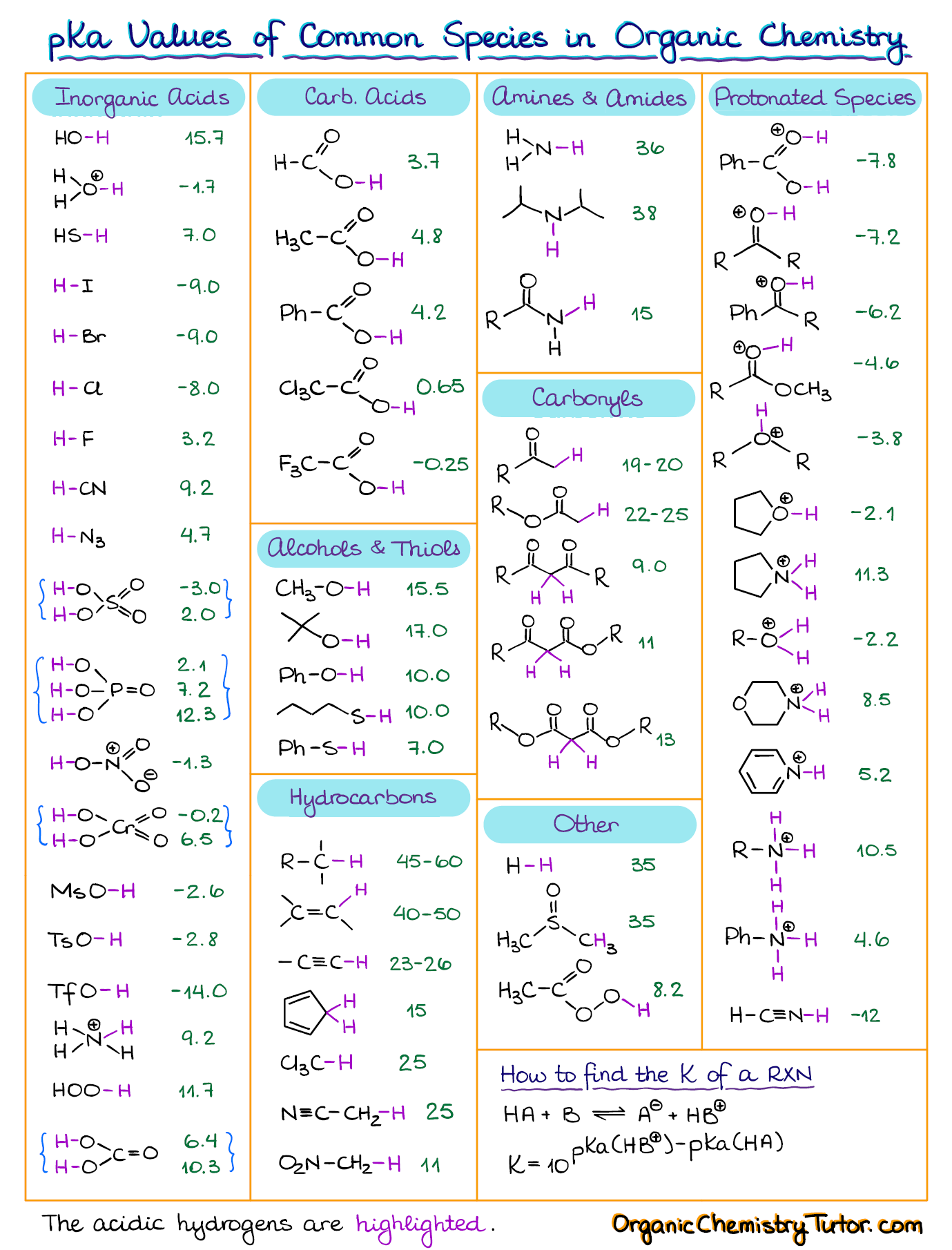
![[Infographic] Comprehensive pKa Chart r/chemistry](https://external-preview.redd.it/K3Snfd3HbLKkQbUsJ8g5GMVBN8te4Altg0_bder8QLE.jpg?auto=webp&s=d6f9b865541ac9cf9c578f5146e380a014396caa)